As the global focus on climate change is increasing, the national carbon neutral carbon peak policy is increasingly implemented. In the process of energy transition, hydrogen, as a kind of clean energy, production, storage and transportation are increasingly difficult. Electrolyzing water for hydrogen production and methanol reforming for hydrogen production, as two common hydrogen production methods, have been widely concerned by both inside and outside the industry. This paper will compare these two hydrogen production methods, discuss their advantages and disadvantages and applicable scenarios, in order to provide reference for the energy transition.
Electrolytic water for hydrogen production
Electrolysis of water to produce hydrogen is a process of using electricity to decompose water (H2O) into hydrogen (H2) and oxygen (O2). This method has the following advantages:
1. Clean and environmental protection: the process of water electrolytic hydrogen production does not involve any combustion reaction, and does not produce harmful gas emissions.
2. High purity hydrogen: electrolysis of water to produce hydrogen can obtain high purity hydrogen, high purity, a little purification can be directly used in fuel cell and other applications.
3. Utilization of renewable energy: Electrolysis of water can be combined with renewable energy (such as solar and wind energy) to realize sustainable utilization of energy by converting renewable energy into hydrogen for storage and utilization.
However, there are some challenges with water lysis:
1. High energy consumption: Electrolysis of water for hydrogen production requires a lot of electricity, and the high power demand for hydrogen makes this type of hydrogen production uneconomical in areas where fossil fuels can generate electricity, and it also has the problem of high emissions.
2. Hydrogen storage and transportation: Hydrogen storage and transportation is relatively difficult, which involves high-pressure hydrogen storage or liquefied hydrogen storage technologies, which increases the complexity and cost of the system.
3. High cost: The current cost of hydrogen hydrogen electrolysis technology is relatively high, mainly from the cost of electrolysis equipment and KWH.

Methanol reforming hydrogen production
Methanol reforming to produce hydrogen is a process of catalyst methanol (CH3OH) through a chemical reaction at high temperature to produce hydrogen. This method has the following characteristics:
1. Convenient storage and transportation: Compared with hydrogen in the gas state, liquid methanol is easier to store and transport, which reduces the cost and safety problems caused by hydrogen storage and transportation.
2. Benefit from the old refueling network facilities: the storage, transportation and methanol filling can make use of the existing liquid fuel infrastructure, without building new hydrogenation stations, which can effectively reduce the infrastructure input required for the energy transition.
3. Wide sources of raw materials and low price: methanol, as a fuel with diversified production methods, can be obtained through biomass synthesis or fossil energy, with a wide range of sources, and is one of the fuels with the largest stock in China.
However, methanol reforming for hydrogen production also faces the following challenges:
a) carbon emission problem: If the methanol production process involves the use of fossil fuels, it has a certain amount of carbon emissions, but the carbon emission closed loop can be realized through the application of green alcohol made from renewable resources.
b) Low purity of hydrogen: the hydrogen produced by methanol reforming may contain certain impurities, which needs to be treated later to obtain high purity hydrogen. However, the online output of high purity hydrogen can be realized by carrying the palladium film purification device.
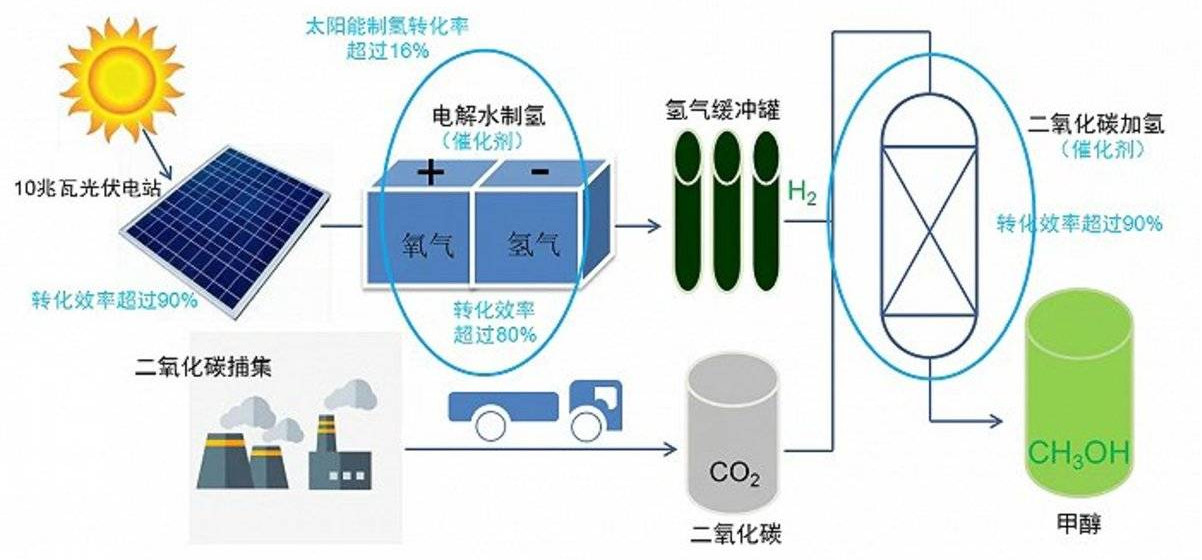
At present, liquid sunshine methanol production technology is available to cope with the carbon emission dilemma of methanol application. Liquid sunlight is the combination of hydrogen produced from solar / wind energy with captured carbon dioxide to produce methanol. The methanol generated by this route can store hydrogen in liquid form, convenient transportation, safe and efficient, and can also achieve closed loop carbon emissions, so as to achieve the goal of carbon neutrality.
For the impurities produced in the process of methanol reforming for hydrogen production, there are some common purification techniques on the market to purify hydrogen:
Pressure molar adsorption (Pressure Swing Adsorption, short) for PSA: PSA is a method of separating impurity gas based on adsorbent. In the PSA process, when hydrogen passes through the adsorbent bed layer, the adsorbent selectively adsorbs other gas components, such as methane and carbon monoxide, so as to realize the purification of hydrogen.
Membrane separation (Membrane Separation): Membrane separation is a process of separating gases by molecular size or solubility using a specific semi-permeable membrane. In the purification process of hydrogen, choosing the appropriate membrane material and operating conditions can separate the impurity gas from the hydrogen.
Normal temperature adsorption (Temperature Swing Adsorption, TSA): TSA uses the adsorption selectivity of different component gas at room temperature to achieve separation. The impurity gas can be separated from the hydrogen by regulating the adsorption rate and affinity of the different gases on the adsorbents.
Usually, methanol hydrogen produces hydrogen in the form of large chemical industry, and through chemical reforming catalytic hydrogen production enters PSA pressure adsorption system for purification, which has certain regional limitations. The hydrogen produced also needs to be stored and transported in the form of high pressure or liquefaction, and the cost problem of storage and transportation end still cannot be solved.
The methanol online hydrogen production system independently developed by Guangdong Nengchuang Technology can effectively solve the problem of hydrogen storage and transportation in methanol hydrogen production in large chemical industry. Meanol online hydrogen production system is to integrate the methanol reforming module and palladium film purification module, to replace the large chemical industry with small equipment, high structural strength, can realize methanol online hydrogen production in projects that need to move or usable small footprint, that is, production and use. Among them, the palladium film purification technology developed by the company can effectively purify the hydrogen containing impurities to the standard purity of hydrogen for fuel cell in a small volume. Based on the current methanol futures price of 2200-2500 yuan, the cost of hydrogen production per kilogram is only 16-18 yuan. At the same time, methanol is also the optimal liquid carrier of hydrogen. Through the online hydrogen production of methanol, the cost of hydrogen at the storage and transportation end can be greatly reduced, so as to reduce the cost of hydrogen for end users.

In conclusion, hydrogen electrolysis and methanol reforming are potential hydrogen production methods. Hydrogen production by electrolysis of water has the advantages of clean environmental protection, high purity hydrogen and renewable energy utilization, which is suitable for areas with abundant renewable energy and stable power supply. Methanol reforming hydrogen production has the advantages of convenient storage and transportation, utilization of infrastructure and wide range of application, and is suitable for promotion and application in areas with relatively developed liquid fuel infrastructure. In practical application, the most appropriate hydrogen production method is selected according to regional renewable energy resources, energy demand, carbon emission reduction targets and application scenarios, so as to promote energy transformation and achieve the goal of carbon neutrality.